Analytical chemistry in space exploration
By Nipun Chandrasiri
To answer some of the biggest questions of life, such as how life began on Earth or if there is life out there, we need to understand what the universe is made up of. From the beginning of the space exploration era, scientific measurements were made to understand the composition of various planetary bodies.
Analytical chemistry, the realm of scientific exploration that includes using instruments and methods to explore the chemical makeup of a sample or a system, is a significant method used in space exploration. Observing the atomic and molecular constitution of distant galaxies or analyzing samples taken from asteroids and planets requires rather specialized instrumental techniques. Presented here are a few of the key analytical chemistry techniques used in space exploration:
Gas Chromatography-Mass Spectrometry (GC-MS)

Gas chromatography (GC) is a widely used technique in analytical chemistry. GC instruments can separate complex chemical mixtures and analyze volatile chemicals with high sensitivity and fast analysis time. When a sample is injected, it’s vaporized and mixed with a carrier gas (mobile phase) and then goes through a stationary phase that interacts with compounds in the mixture in different ways, allowing for their separation. In different columns with different functional groups, porous materials are used as the stationary phase which can separate the molecules in the mobile phase according to their affinity to the column. The elution time of a chemical species also can be controlled by changing the temperature. After the separation, molecules are analyzed using detectors like Mass Spectrometry (MS), a Flame Ionization Detector (FID), a Nitrogen Phosphorus Detector (NPD), or an Electron Capture Detector (ECD).
Mass spectroscopy (MS) detectors can be coupled with GC, and they can give both quantitative and qualitative information about the sample. MS detectors have sensitivity ranges as low as 1-10 ng or 1-10 pg for some species.
GC-MS can be used to determine atmospheric composition by detecting many gases such as CO2, N2, Ar, CH4, NH3, H2S, CO, H2, NO, SO2. GC-MS can also be used on a variety of samples collected on planetary surfaces. The presence of volatile organic compounds, hydrocarbons, amino acids, porphyrins, organic acids, alcohols, aldehydes, and ketones — all of which can be strong indicators for biological activity — can be detected using GCMS.
Spectroscopy
Spectroscopy measures how matter interacts with electromagnetic radiation. Radiation has a range of wavelengths, from kilometers to picometers, and spans from radio waves and microwaves at longer wavelengths to x-rays and gamma rays at shorter wavelengths. Every molecule and even element has a unique way of interacting with radiation. These interactions can be used to detect many organic and inorganic compounds and even elements in or on planetary bodies using remote sensing instruments. Many spacecraft have been built with instruments that are sensitive to wavelengths in infrared, visible, ultraviolet radiation, and even the more extremes. Based on the type of interactions with matter, spectroscopic measurement can be classified into three general categories.
- Absorption spectroscopy – Measures the absorption of electromagnetic radiation as a function of wavelength or frequency. Electrons in the ground state of molecules will absorb the energy of electromagnetic radiation and reach higher energy states. Since molecules have unique absorption wavelengths, dips in the specific wavelength will indicate the presence of a particular molecule. (ex – for O2 – 759-771 nm)
- Emission spectroscopy – Measures the electromagnetic radiation emitted when electrons shift from excited states to ground states. This gives unique emission wavelengths that can be used to determine the composition of hot objects such as stars.

- Scattering spectroscopy – Measures the amount of scattering of the electromagnetic radiation when it interacts with molecules at certain wavelengths.
Some types of spectroscopy instruments
Ultraviolet Spectrometer (UVS) – Covers the wavelength range of 10 nm to 320 nm looking at planetary atmospheres and interplanetary space. The chemical composition, densities, and temperatures of the interstellar medium and stars can be determined using this technique. Identifies the surface composition and structure of atmospheres, determine volatile gas loss rates, compositions and dynamics of exospheres, and search for ice surface layers.
Near-Infrared Mapping Spectrometer (NIMS) – Maps morphological features, while simultaneously determining their composition and mineralogy, providing data to investigate the evolution of surface geology.
Tunable Laser Spectrometer (TLS) – Measures the absorption of light at specific wavelengths. TLS can be used to measure concentrations of gases such as methane, carbon dioxide, and water vapor in the Martian atmosphere.
X-ray Spectroscopy – Uses absorption and emission in the X-ray range of the electromagnetic spectrum ( 0.01 nm to 10 nm). It can be used to determine the composition and conditions of high-temperature plasmas of galaxies and to investigate neutron stars and black holes.
Wet Chemistry Instruments
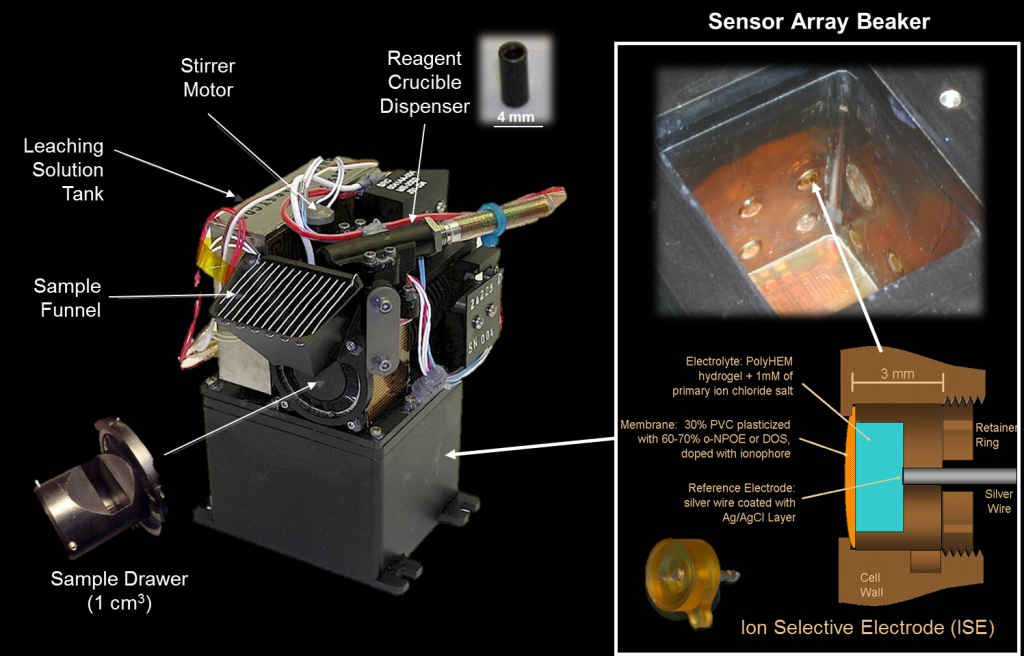
Wet chemistry is another analytical method that can be used qualitatively or quantitatively to determine the chemical properties of a sample. Wet chemistry analysis includes colorimetry, gravimetry, and titrimetry. The Phoenix mission carried out the first wet chemistry experiments on another planet (Mars). Some wet chemistry instrumental techniques include1 :
Ion-selective electrodes (ISE) – Used to determine the presence of ions in a sample, such as Ca2+, Mg2+, K+, Na+, NH4+, Cl–, Br–, I–, NO3-, ClO4-, and SO42-.
pH meters – Indicate the degree of concentration of hydrogen ions in a sample.
Conductivity meters – Used to determine the composition of ionic species in a sample.
Anodic stripping voltammetry (ASV) instruments – Used for the detection of heavy metal ions in solution
Chronopotentiometry (CP) – This allows for the independent determination of ions such as chloride, bromide, and iodide.
Cyclic voltammetry (CV) – Used for identifying and analyzing possible reversible and irreversible redox couples.
Conclusion
Using analytical instruments in space requires specially modified hardware. It must be small and low mass, have low power consumption, provide high mechanical and shock strength, and have the ability to work in extremely harsh environments. Even though it’s challenging to use these techniques in space exploration, analytical chemistry is a must-have tool to study the solar system and beyond.
Programs like Planetary Instrument Concepts for the Advancement of Solar System Observations (PICASSO), Maturation of Instruments for Solar System Exploration (MatISSE), and Instrument Concepts for Europa Exploration (ICEE) support the development and advancement of analytical instruments for space exploration. In future missions, more capable and sensitive instruments will pave the way to understand life throughout the cosmos.
References:
- Analytical Chemistry in Astrobiology: Kenneth Marshall Seaton, Morgan Leigh Cable, and Amanda Michelle Stockton Analytical Chemistry 2021 93 (15), 5981-5997DOI: 10.1021/acs.analchem.0c04271
- McElhoney, K. M.; O’Neil, G. D.; Kounaves, S. P. Extraterrestrial. In Environmental Analysis by Electrochemical Sensors and Biosensors; Springer New York: New York, NY, 2014; pp 131–151.
- https://solarsystem.nasa.gov/basics/chapter12-1/
- http://planetary.chem.tufts.edu/Phoenix/WetChemLab.html
- https://hubblesite.org/mission-and-telescope/instruments
- https://www.azolifesciences.com/article/X-Ray-Spectroscopy-An-Overview.aspx
About the author: After completing my undergraduate degree in Chemistry honors, I am currently working as a teaching assistant at the University of Peradeniya, Sri Lanka. Also working as a RA at BMSIS. I am planning to apply for graduate studies in a few weeks.
